Electronic Instructions for Use (eIFU)
Some of our products are supplied without printed instructions for use (IFU). Here you can easily search, view, download or print the IFU in the current version.
-
eIFU for Implants
-
- eIFU for Beznoska Knee Joint
-
- eIFU for Beznoska Hand
-
eIFU for Reusable instruments
Search by article number (REF) is possible
Need an old version of the IFU? Visit Archived eIFU
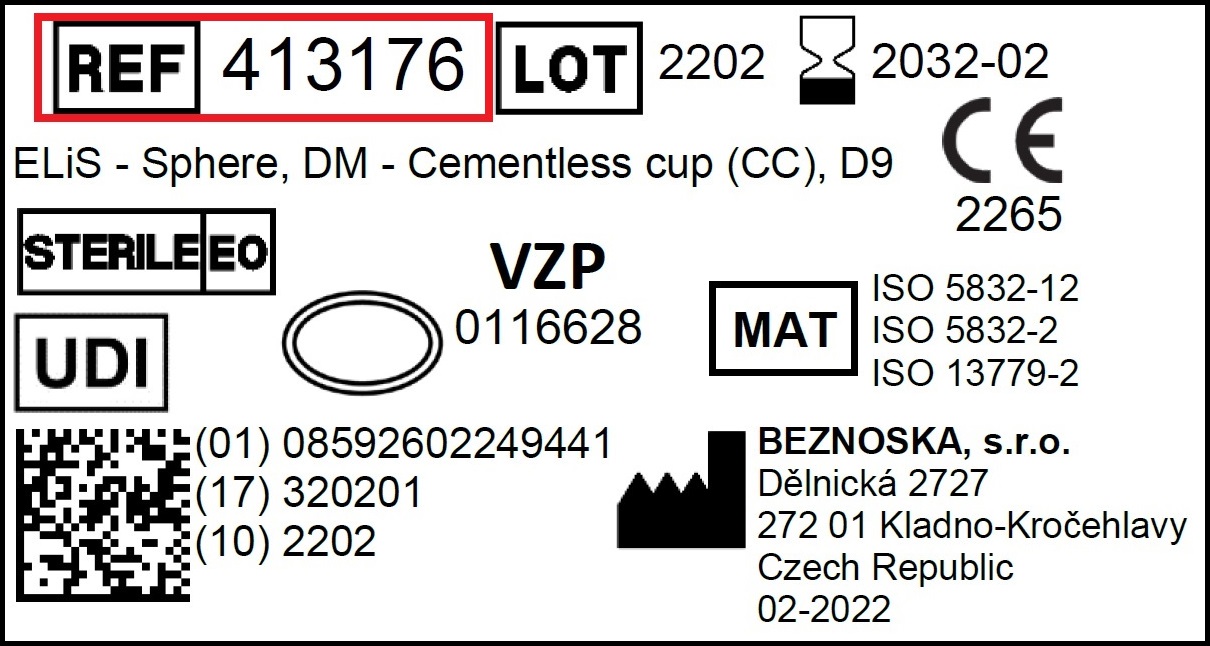
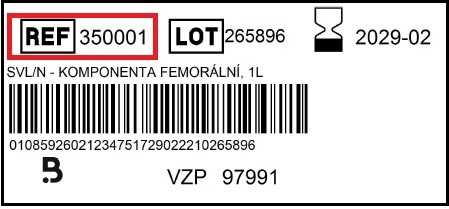
Where can I find REF number?
The REF number of our products can be found in the product label, which is placed on the packaging as well as on the inside the.
 |
|
 |
Why electronically?
Commission Implementing Regulation (EU) 2021/2226 of 14 December 2021 laying down detailed rules for the implementation of Regulation (EU) 2017/745 of the European Parliament and of the Council as regards electronic instructions for use of medical devices allows manufacturers to provide instructions for use medical devices exclusively in digital form. However, if you still need the IFU in printed paper form, we will be happy to send it to you free of charge. You can easily make a request for specific document by contacting us by e-mail or phone.
How to recognize products with eIFU
The transition to electronic instructions for use and leaflets is taking place gradually. You may still come across products that will contain a leaflet or instructions for use (IFU) in paper form, while other products may no longer contain them. The existence of an eIFU is indicated by a symbol on the product label.
Electronic instructions for use (eIFU) are issued for products bearing this symbol on the label
Electronically and ecologically
The transition to an electronic form of documents will save several tons of paper every year, which would be used for printed package leaflets and instructions for use. By reducing the amount of used paper we are helping to protect our environment.